Contract Researchers
ARM Company Customizations
Some of GDM's global corporate clients use customized ARM study definitions and validation lists. They also provide their customized study definitions to independent contract researchers. This approach ensures uniformity and enhanced data management.
Since independent contract researchers often conduct research studies for more than one company, GDM has a customized install for company customizations. (See below for installation instructions.)
Each company’s customizations are stored separately, so multiple companies’ customizations can reside on one ARM program. ARM automatically keeps separate settings for each customization, and refers to each as a “Settings profile.”
A contractor or cooperator may request sponsor customizations (pdf) within ARM. After approval, use the method described below in "Installation Directions" to install approved sponsor customizations.
Note: Always sent trials to sponsor using File - Sent to - External Sponsor/Cooperator, to ensure the sponsor receives all trial information.
Installation Directions
|
Opening Studies without Customized Company Files
A few research sponsors do not distribute their ARM customizations outside of their company. However, a contract researcher may occasionally receive a study created with customized study definitions that ARM cannot open because the study definitions are not available in standard ARM installations.
The message "Study definition 'xxxxxx.def' is not present in a known study definition directory. Can ARM search the drives on your computer to locate this study definition?" displays.
If not found, another message displays: "Study definition 'xxxxxx.def' not in current definition directory. Select the directory containing the definition next." Click the OK button.
If the company representative who sent the ARM study is not providing you with their customized study definitions, then on the "Select Study Definition" wizard choose: "Myself or another sponsor (standard GDMdef)":
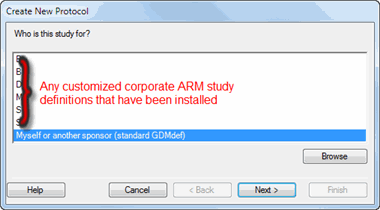
Click the Next button.
Choose G-All7 for most studies, or G-Seed7 for a pure variety testing study:
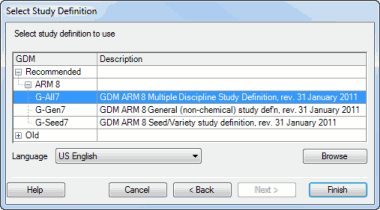
Click the Finish button.
Please ensure you are using the most recent ARM maintenance update, since many times the most recent update includes changes to improve compatibility when opening studies into a GDM study definition.
Sending Trial Results to Sponsor
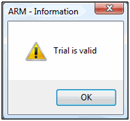

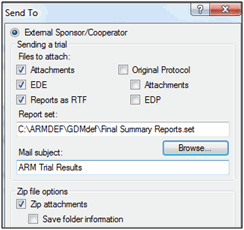
This will:
- Validate the trial
- Include the ARM trial so all original data is included
- Include attachments, providing all supplemental information
- Include reports printed to *.rtf for a quick overview of trial results